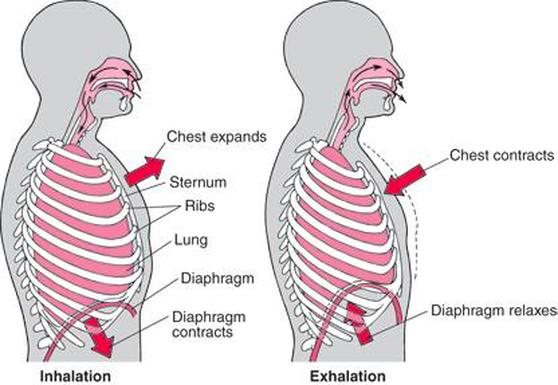
Introduction to Scientific Measurement:
Definition: Scientific measurement involves the systematic determination of quantities using standardized procedures and instruments. It is fundamental to scientific inquiry, providing accurate and reliable data for experimentation, analysis, and communication.
Key Concepts in Scientific Measurement:
- Accuracy vs. Precision:
Accuracy refers to the closeness of a measured value to the true or accepted value, while precision relates to the consistency and reproducibility of repeated measurements.
- Significant Figures:
Definition: Significant figures are the digits in a measurement that contribute to its precision. Rules govern how to determine and use significant figures in calculations.
- Error Analysis:
Definition: Error analysis involves assessing and quantifying the uncertainties associated with measurements. Understanding and minimizing errors enhance the reliability of experimental results.
SI Units: International System of Units:
Definition:
The International System of Units (SI) is a globally accepted system of measurement that provides a standardized framework for expressing physical quantities. It consists of seven base units, from which all other units are derived.
Seven Base SI Units:
Meter (m): unit of length. The distance traveled by light in a vacuum during a specific time interval defines the meter.
Kilogram (kg): unit of mass. Initially defined by a platinum-iridium alloy cylinder, the kilogram is now defined by fundamental constants.
Second (s): unit of time. Defined by the vibration of cesium atoms.
Ampere (A): unit of electric current. Defined by the flow of electric charge.
Kelvin (K): unit of temperature. Defined by the triple point of water.
Mole (mol): unit of amount of substance. One mole contains Avogadro’s number of entities (atoms, molecules, etc.).
Candela (cd): unit of luminous intensity. Defined by a specific frequency of light.
Derived SI Units:
- Newton (N): unit of force, derived from mass and acceleration (1 N = 1 kg·m/s²).
- Joule (J): unit of energy, derived from force and distance (1 J = 1 N·m).
- Watt (W): unit of power, derived from energy and time (1 W = 1 J/s).
Practical Applications of SI Units:
- Science and Engineering: SI units provide a standardized language for scientists and engineers, facilitating precise communication and collaboration.
- International Trade and Commerce: SI units simplify transactions and measurements in global trade, ensuring consistency and accuracy.
Conversions and Prefixes:
- Prefixes: Prefixes such as kilo-, milli-, and micro- indicate multiples or fractions of base units.
- Unit Conversions: Understanding and applying conversion factors between different units is crucial for versatility in scientific measurements.