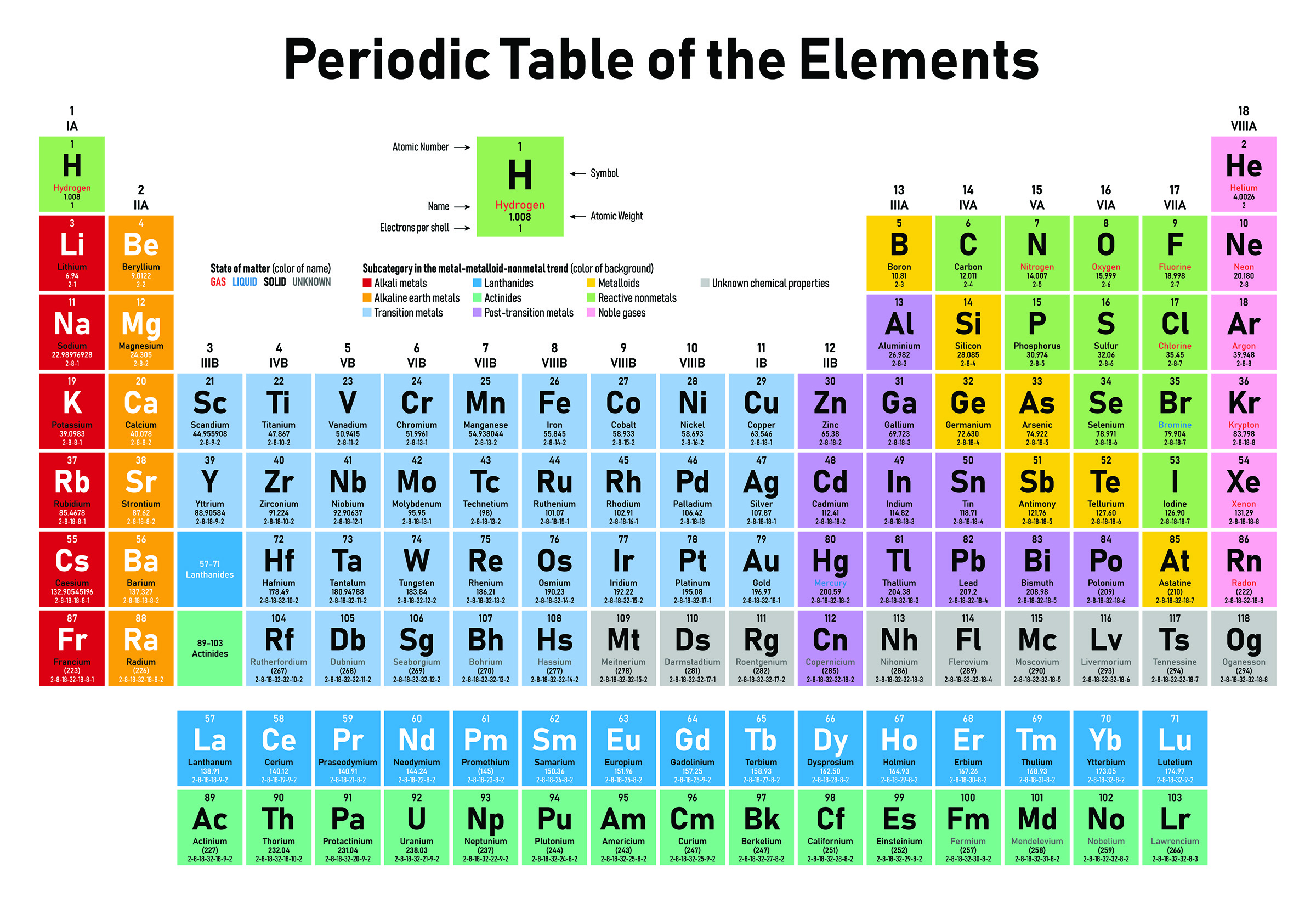
The Periodic Table stands as a monumental achievement in the realm of chemistry, offering a systematic arrangement of elements that unveils the intricate relationships and properties shared by these fundamental building blocks of matter. This exploration delves into the history and structure of the Periodic Table, sheds light on its significance, and provides an overview of each element and its unique attributes.
Historical Perspective:
The genesis of the Periodic Table can be traced back to the 19th century, when various scientists, including Antoine Lavoisier and John Newlands, attempted to organize the known elements based on their properties. However, it was Dmitri Mendeleev who made a groundbreaking contribution. In 1869, Mendeleev arranged the elements in a systematic order based on increasing atomic mass, grouping them by similar properties. Notably, he left gaps for yet-to-be-discovered elements, predicting their properties with remarkable accuracy.
The Modern Periodic Table:
The modern Periodic Table is organized based on atomic number, the number of protons in an atom’s nucleus. Elements are arranged in ascending order of atomic number, which leads to a periodic repetition of properties.
Periods: Rows in the Periodic Table are called periods. Each period represents a new energy level or shell for electrons.
Groups: Columns in the Periodic Table are called groups or families. Elements in the same group share similar chemical properties due to having the same number of electrons in their outermost shell.
KEY REGIONS OF THE PERIODIC TABLE:
Transition Metals: Found in the center of the Periodic Table, transition metals exhibit a wide range of oxidation states and are known for their metallic properties.
Alkali Metals: Group 1 elements, including lithium, sodium, and potassium, are highly reactive metals that readily lose electrons.
Alkaline Earth Metals: Group 2 elements, such as magnesium and calcium, are reactive metals with distinctive properties.
Halogens: Group 17 elements, such as fluorine and chlorine, are highly reactive nonmetals that readily form compounds.
Noble Gases: Group 18 elements, including helium and neon, are inert gases that generally do not form compounds due to their stable electron configurations.
OVERVIEW OF SELECTED ELEMENTS:
Hydrogen (H): The lightest and most abundant element in the universe, hydrogen is a colorless, odorless gas. It forms diatomic molecules (H₂) and plays a crucial role in various chemical processes, including the formation of water.
Oxygen (O): Essential for life, oxygen is a diatomic gas that supports combustion and respiration. It is a vital component of water and various organic compounds.
Carbon (C): The building block of life, carbon is the foundation of organic chemistry. It forms diverse compounds, including hydrocarbons and carbohydrates.
Iron (Fe): A transition metal, iron is vital for human health, playing a central role in hemoglobin for oxygen transport in blood.
Gold (Au): A precious metal, gold has been prized for its rarity and aesthetic qualities throughout history. It is known for its malleability and unreactivity.
Uranium (U): A radioactive element, uranium is crucial in nuclear reactions and the production of nuclear energy.
Helium (He): An inert noble gas, helium is known for its low boiling point and is commonly used in balloons and as a coolant in various applications.
Trends in the Periodic Table:
Atomic Size: Atomic size generally decreases across a period and increases down a group. This trend is attributed to changes in effective nuclear charge and electron shielding.
Ionization Energy: Ionization energy is the energy required to remove an electron from an atom. It generally increases across a period and decreases down a group.
Electronegativity: Electronegativity is the tendency of an atom to attract electrons in a chemical bond. It generally increases across a period and decreases down a group.
Periodic Trends in Selected Elements:
Fluorine (F): A highly electronegative halogen, fluorine readily forms compounds and is a key component in fluoridated compounds for dental health.
Sodium (Na): An alkali metal, sodium is highly reactive and reacts vigorously with water to produce sodium hydroxide and hydrogen gas.
Chlorine (Cl): A halogen, chlorine is widely used for disinfection and is a crucial component in the production of PVC (polyvinyl chloride) plastics.
Argon (Ar): A noble gas, argon is inert and is used in various applications, including welding and as a filler gas in incandescent light bulbs.
The Periodic Table serves as a roadmap to the universe of elements, guiding scientists in their exploration of the fundamental constituents of matter.
From the early attempts to organize elements based on properties to the sophisticated modern Periodic Table, this tool has not only facilitated our understanding of the relationships between elements but also guided scientific discoveries.
Each element, with its unique properties and contributions to chemistry and beyond, adds to the symphony of matter that defines the natural world.
As we continue to delve into the depths of the Periodic Table, we unlock new insights into the nature of elements and their roles in shaping the physical and chemical landscapes of our universe.