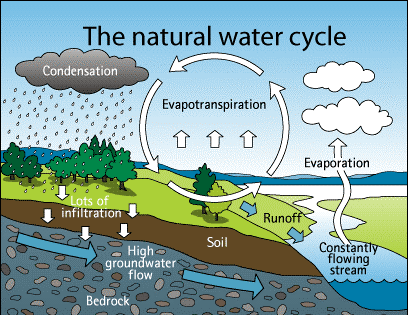
Naming compounds is an essential part of chemistry. In order to understand the properties and reactions of compounds, it is necessary to identify them accurately. Naming compounds can be challenging because there are rules and conventions that must be followed. In this essay, we will simplify complex concepts about naming compounds and provide a clear understanding of the fundamental principles involved.
The Basics of Naming Compounds
The first step in naming a compound is to identify the elements that make up the compound. Compounds are made up of two or more elements that are chemically bonded together. The name of the compound is based on the elements that make it up. For example, water is a compound made up of hydrogen and oxygen. The chemical formula for water is H2O.
Types of Compounds
There are two main types of compounds: ionic compounds and covalent compounds. Ionic compounds are formed by the transfer of electrons between atoms, while covalent compounds are formed by the sharing of electrons between atoms.
Ionic Compounds
Ionic compounds are made up of a metal and a non-metal. When the metal and non-metal bond, the metal loses electrons and the non-metal gains electrons. This creates ions with opposite charges that are attracted to each other, forming a compound. The name of an ionic compound is based on the elements that make it up. The metal is listed first, followed by the non-metal. For example, sodium chloride is an ionic compound made up of sodium and chlorine. The chemical formula for sodium chloride is NaCl.
Covalent Compounds
Covalent compounds are made up of non-metals. When non-metal atoms bond, they share electrons. The name of a covalent compound is based on the number of atoms of each element that make it up. The prefixes used in naming covalent compounds indicate the number of atoms of each element. For example, carbon dioxide is a covalent compound made up of one carbon atom and two oxygen atoms. The prefix for one is “mono,” so the name of the compound is carbon monoxide.
Polyatomic Ions
Polyatomic ions are groups of atoms that are covalently bonded together and have an overall charge. Polyatomic ions are treated like single atoms when naming compounds. For example, the sulfate ion is SO4-2. When naming a compound that contains the sulfate ion, the sulfate ion is named first, followed by the name of the other ion. For example, magnesium sulfate is a compound made up of magnesium and the sulfate ion. The chemical formula for magnesium sulfate is MgSO4.
Naming compounds is an essential part of chemistry. By understanding the principles of naming compounds, we can accurately identify and describe the properties and reactions of compounds. The key to naming compounds is to identify the elements that make up the compound and to follow the rules and conventions of naming. With practice, naming compounds can become second nature, and we can gain a deeper understanding of the complex world of chemistry.