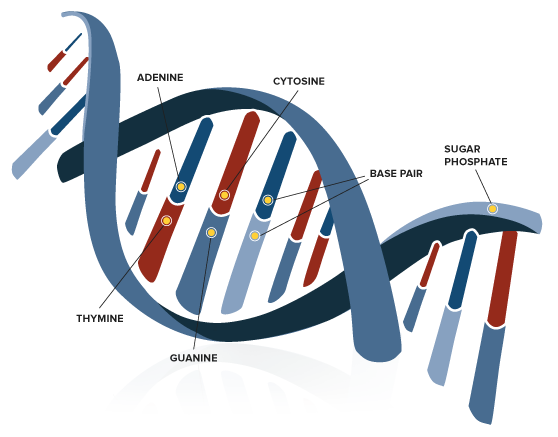
Chemical reactions are the building blocks of chemistry. They are the changing processes that turn substances into new things. The goal of this in-depth study is to reveal the mysteries of chemical reactions by revealing the basic rules and methods that control these changing events.
Definition of Chemical Reactions:
Chemical reactions can be defined as the rearrangement of atoms in molecules to create new substances with distinct properties. The reactants, the original substances entering the reaction, undergo a series of interactions leading to the formation of products.
The law of conservation of mass, which states that the total mass of the reactants equals the total mass of the products, is a fundamental principle of mass conservation.
Types of chemical reactions:
Chemical reactions manifest in various forms, each categorized based on the nature of the changes occurring. Common types include:
Combustion reactions involve the rapid reaction of a substance with oxygen, often accompanied by the release of heat and light.
Synthesis Reactions: Formation of a new compound from simpler reactants.
Decomposition reactions: the breakdown of a compound into simpler substances.
Single and Double Displacement Reactions: exchange of ions between reactants in single displacement and exchange of ions between two compounds in double displacement.
Chemical Equations:
Chemical reactions are symbolically represented through chemical equations. Reactants are denoted on the left side of the arrow, and products are on the right. Coefficients represent the stoichiometric coefficients, indicating the relative quantities of substances involved. Balancing chemical equations ensures that the law of conservation of mass is upheld.
Factors Influencing Reaction Rates:
Numerous factors affect the reaction rate or the speed at which a chemical reaction occurs:
Concentration: Higher concentrations of reactants typically lead to faster reaction rates.
Temperature: Increased temperature often accelerates reaction rates by providing more kinetic energy to molecules.
Surface Area: In the case of solid reactants, a greater surface area enhances the likelihood of collisions and reactions.
Catalysts: substances that alter the reaction mechanism, lowering the activation energy and expediting the reaction without being consumed in the process.
Reaction Mechanisms:
Chemical reactions are often complex processes involving a sequence of steps. The reaction mechanism elucidates the detailed pathway through which reactants transform into products. Elementary steps, intermediates, and the overall reaction order contribute to our understanding of these intricate processes.
Thermodynamics of Chemical Reactions:
Thermodynamics, the study of energy changes in systems, plays a crucial role in understanding chemical reactions. The concepts of exothermic and endothermic reactions, enthalpy changes, and Gibbs free energy help quantify the energy changes associated with reactions. The sign of the Gibbs free energy change determines the spontaneity of a reaction.
Chemical Kinetics:
Chemical kinetics explores the rate of chemical reactions and the factors influencing it. Rate laws express the relationship between reactant concentrations and reaction rates, providing insights into the reaction’s order with respect to each reactant. Activation energy, the energy required to initiate a reaction, is a key parameter in understanding reaction kinetics.
Redox Reactions:
Redox (reduction-oxidation) reactions involve the transfer of electrons between reactants. Oxidation involves the loss of electrons, while reduction involves the gain. Redox reactions are pivotal in various biological processes, industrial applications, and energy storage systems.
Applications of Chemical Reactions:
The practical applications of chemical reactions span diverse fields:
Chemical Industry: Synthesis of a myriad of products, from pharmaceuticals to polymers.
Energy Production: Combustion reactions in fossil fuels and redox reactions in batteries.
Environmental Processes: Decomposition reactions in waste treatment and natural decay.
Chemical processes are what chemistry is all about; they make it possible for matter to change over time. Deep knowledge of chemical reactions opens up the alchemy of matter, from the microscopic world of how reactions work to the macroscopic world of how they affect our daily lives. Not only do we learn more about this fascinating subject, but we also gain a better understanding of how the world around us is formed by the complex dance of atoms and molecules.