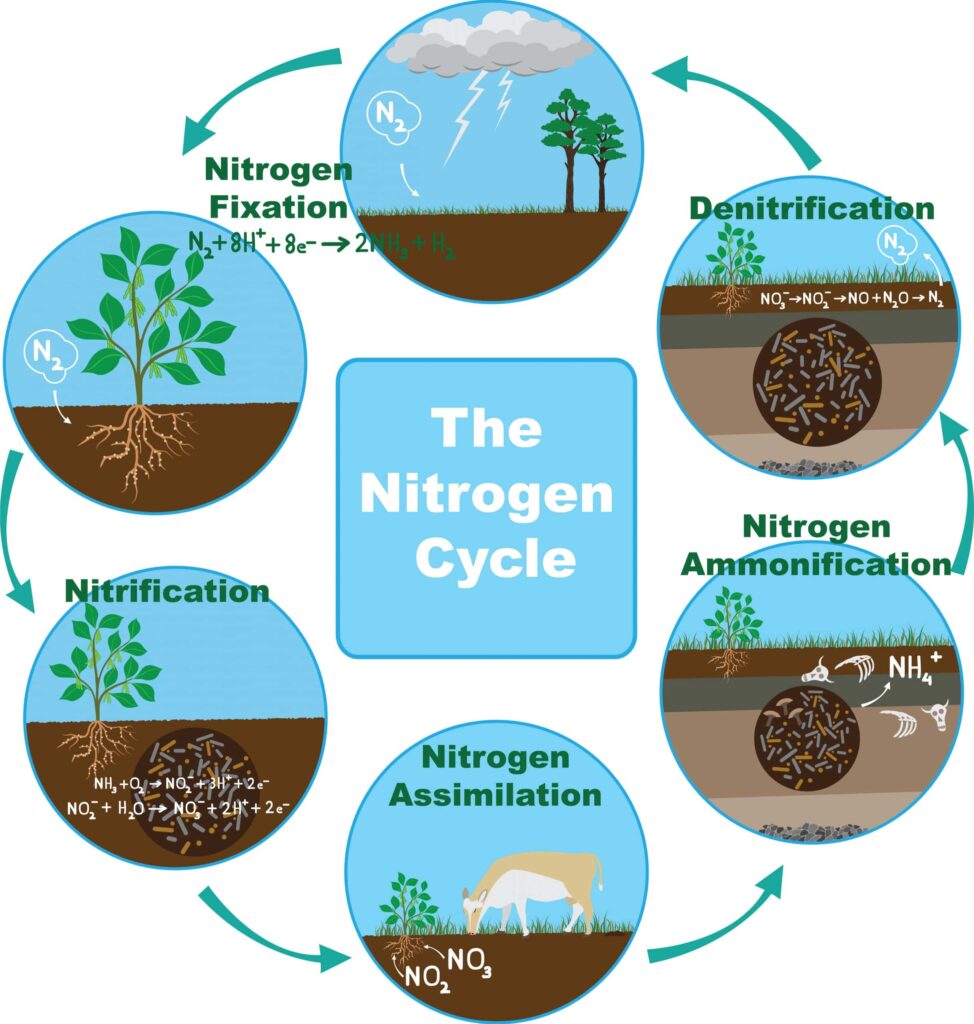
A very important biogeochemical process called the nitrogen cycle flows through Earth’s ecosystems and affects the supply of nutrients that living things need. This study looks into the complicated nitrogen cycle, revealing its different stages, important players, and the huge effect it has on the balance of nature.
Introduction to the Nitrogen Cycle:
The nitrogen cycle encompasses a series of interconnected processes that govern the transformation of nitrogen into various forms within the biosphere. Nitrogen, a crucial component of proteins and nucleic acids, is essential for the growth and development of living organisms. The cycle involves the transformation of atmospheric nitrogen (N2) into forms that plants and other organisms can use, with nitrogen returning to the atmosphere to complete the circle.
Nitrogen Fixation:
The atmospheric nitrogen (N2) that constitutes nearly 78% of Earth’s atmosphere is inert and unavailable for direct use by most organisms. Nitrogen fixation, the first step in the nitrogen cycle, is the process by which atmospheric nitrogen is converted into ammonia (NH3) or nitrate (NO3-) by nitrogen-fixing bacteria. These bacteria, often associated with the roots of leguminous plants like peas and beans, have the unique ability to break the strong triple bond of atmospheric nitrogen, making it accessible to the ecosystem.
Ammonification:
Ammonification involves the conversion of organic nitrogen compounds into ammonia. Decomposer organisms, such as bacteria and fungi, that break down organic matter from dead plants and animals and release ammonia into the soil are the main drivers of this process. The ammonia produced serves as a critical source of nitrogen for subsequent stages in the nitrogen cycle.
Nitrification:
Nitrification is a two-step process that transforms ammonia into nitrite (NO2-) and then into nitrate. Ammonia-oxidizing bacteria, such as Nitrosomonas, oxidize ammonia to nitrite, while nitrite-oxidizing bacteria, like Nitrobacter, further convert nitrite to nitrate. Nitrate is a form of nitrogen readily taken up by plants and is a key component of many fertilizers.
Assimilation:
Assimilation involves the incorporation of inorganic nitrogen compounds (ammonium, nitrate) into organic molecules by plants and microorganisms. Plants assimilate nitrogen by absorbing nitrate or ammonium from the soil through their roots. Animals, in turn, obtain nitrogen by consuming plants or other animals. The assimilated nitrogen becomes part of amino acids, proteins, and nucleic acids, contributing to the growth and development of living organisms.
Denitrification:
Denitrification is a microbial process that converts nitrate back into atmospheric nitrogen (N2) or nitrous oxide (N2O). Denitrifying bacteria, such as Pseudomonas and Paracoccus, play a crucial role in this process. Denitrification helps regulate the nitrogen levels in soils and prevents the accumulation of excess nitrogen, which could lead to environmental issues such as water pollution.
Importance of Nitrogen in Ecosystems:
Nitrogen is a fundamental element for the structure and function of living organisms. It is a key component of proteins, essential for cellular structure and function, and nucleic acids, vital for genetic information. The availability of nitrogen in ecosystems directly influences the growth of plants, the health of ecosystems, and the productivity of agricultural systems.
Human Impacts on the Nitrogen Cycle:
Human activities, particularly in agriculture and industry, have significantly altered the nitrogen cycle. Excessive use of nitrogen-based fertilizers can lead to nitrogen runoff, polluting water bodies and causing issues like eutrophication.
Combustion processes, such as those in vehicles and power plants, release nitrogen oxides into the atmosphere, contributing to air pollution and acid rain. Understanding these anthropogenic impacts is crucial for developing sustainable practices that minimize the negative consequences for the nitrogen cycle and the environment.
Nitrogen in the atmosphere:
While the nitrogen cycle primarily operates within ecosystems, a significant portion of Earth’s atmosphere contains atmospheric nitrogen (N2). Nitrogen gas is inert and unreactive under normal conditions, and its conversion into reactive forms through processes like nitrogen fixation is critical for maintaining the balance of nitrogen availability in ecosystems.
Future Perspectives and Research:
Ongoing research aims to enhance our understanding of the nitrogen cycle, particularly in the context of global climate change. Climate-induced shifts in precipitation patterns, temperature, and land use may influence nitrogen cycling processes, potentially leading to unforeseen consequences for ecosystems. Interdisciplinary studies that integrate ecological, microbiological, and climatological perspectives are essential for addressing these complexities.
The nitrogen cycle is a complex and changing process that keeps life going on Earth. The nitrogen cycle is very important for keeping the environment balanced. It starts with nitrogen in the air and ends with it being absorbed into biological molecules. Understanding the nitrogen cycle better is important for promoting sustainable practices and reducing the damage to ecosystems and world nutrient cycles as we deal with the problems caused by humans and changes in the environment.






